Database of luminescent minerals
SPHALERITE
Chemical formula: (Zn,Fe)S
Family: Sulfide
Status: IMA-GP
Crystal system : Isometric
Display mineral: OUI
Associated names (luminescent varieties, discredited names, synonyms, etc.): brunckite, cleiophane, schalenblende, blende,
Luminescence:
Longwave UV (365nm) colors: |
Orange , Pale Yellow , Yellow , Orangy yellow , Greenish Yellow , Yellowish , | ||
Intensity LW:Strong | |||
Midwave UV (320nm) colors: |
Orange , Pale Yellow , Yellow , Orangy yellow , Greenish Yellow , Yellowish , | ||
Intensity MW:Strong | |||
Shortwave UV (254nm) colors: |
Orange , Pale Yellow , Yellow , Orangy yellow , Greenish Yellow , Yellowish , | ||
Intensity SW:Medium | |||
Daylight picture

SPHALERITE;
Franklin Mine, Franklin Mining District, Sussex Co., New Jersey, USA
Photo and Copyright:
Middleearthminerals.com
Used with permission of the author
Longwave (365nm) picture
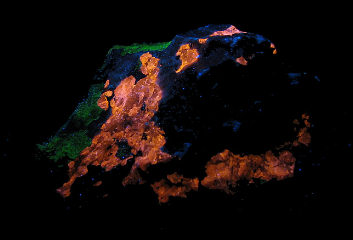
SPHALERITE;
Franklin Mine, Franklin Mining District, Sussex Co., New Jersey, USA
UVLW, Photo and Copyright:
Middleearthminerals.com
Used with permission of the author
Shortwave (254nm) picture
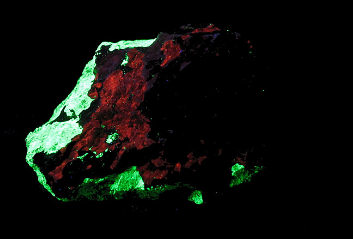
SPHALERITE;
Franklin Mine, Franklin Mining District, Sussex Co., New Jersey, USA
UVSW, Photo and Copyright:
Middleearthminerals.com
Used with permission of the author
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Pale Yellow | UV moyens (320 nm): | Pale Yellow | UV courts (254 nm): | Pale Yellow |
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
synonyme: blende ; brunckite = colloïdal sphalerite ; variety cleiophane: orange SW and LW;
Activator(s) and spectrum:
Activator(s): Mn2+ , Cu+,
Peaks in the spectrum (nm):
Mn2+ replacing Zn2+ : large band peaking at 595 nm VZn-Cl- : 450-460-470nm Cu+-Ga3+, Cu+-In3+ repl Zn2+ : 640-670-675nm
No spectrum yet
Comments on spectrum and activators:
Ed. Becqurel studied the decay of the phosphorescence of the different component (bands) of the spectrum of ZnS, proposing a number of mathemetical expression to interpret this phenomenon (1860). One of the oldest studies on activator in zinc sulphide was made by H. Grune (1904). He noted that a small amont of manganese in ZnS produced an orange luminescence and triboluminescence in a very similar way as the natural mineral. Jorrissen and Ringe (1904) also studied zinc sulfide fluorescence. The influence of the irradiation by a red light and infrared radiations upon the photoluminescence has been studied by C. Peirce (1906) under a variety of conditions. Nichols found the presence of 48 bands/peaks in the spectrum coinciding in part with thallium, Erbium, Ytterbium. [after De Ment, 1949]
Best localities for fluorescence (*):
- Sterling Hill mine (Franklin), Ogdensburg, Sussex county, New jersey, USA;
- Massive sphalérite in Baryte matrix, Hot Springs, Madison County, North Carolina, USA;
- Horn Silver Mine, Frisco, San Francisco District (Frisco District), San Francisco Mts, Beaver Co., Utah, USA;
- Bisbee, Warren District, Mule Mts, Cochise Co., Arizona, USA;
- Luccheti Quarry, Carrara area, Tuscany, Italy (xl in marble fluorescing yellow);
- Hasselhojden, Grythyttan, Hällefors, Västmanland, Sweden;
- Las Manforas Mine, Camaleño, Icos de Europa, Cantabria, Spain (red LW);
- Sweet Home Mine, Mount Bross, Alma Mining District, Park Co., Colorado, USA (better known for his rhodochrosite crystals, sometimes sphalerite (orange LW) associated with fluorine (blue LW) comes from this mine)
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Mt St Hilaire Website: http://www.saint-hilaire.ca ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- Influence de la structure cristallographique sur les spectres optiques des ions de la série du fer dans ZnS, T. Buch, A. Geoffroy et B. Lambert, Journal de Physique, 1974
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2
- Fluorescent Sphalerite and Wurtzite from the Carrara Area, Antonello Dallegno, Guido Mazzoleni in Journal of the Fluorescent Mineral Society, vol 33, 2013
- http://www.minerant.org/archief/fluo-Belgie.pdf
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
Images:
- Hasselhojden, Sweden: http://www.mindat.org/photo-174166.html
- Spain: http://www.mindat.org/photo-484308.html
- Sweet Home Mine, Colorado, USA: https://www.mindat.org/photo-996115.html
- Triboluminescence: https://www.mindat.org/photo-796260.html
Videos:
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Sphalerite
http://www.mindat.org/show.php?name=Sphalerite
 http://webmineral.com/data/Sphalerite.shtml
http://webmineral.com/data/Sphalerite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.