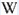Database of luminescent minerals
SCHEELITE
Chemical formula: CaWO4
Family: Tungstates, Molybdates
Status: IMA-GP
Crystal system : Tetragonal
Display mineral: OUI
Associated names (luminescent varieties, discredited names, synonyms, etc.): cuproscheelite, molybdoscheelite,
Luminescence:
Longwave UV (365nm) colors: |
Violet Pink , White , Orangy yellow , Orange , Violet red , | ||
Intensity LW:Very weak | |||
Midwave UV (320nm) colors: |
Red , Orange Red , Red , Violet red , | ||
Intensity MW:Medium | |||
Shortwave UV (254nm) colors: |
Bluish White , White , Yellowish White , Pinkish White , Pale Yellow , Tawn , Yellowish , | ||
Intensity SW:Very Strong | Frequency SW:Always | ||
Daylight picture

SCHEELITE, China
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Shortwave (254nm) picture

SCHEELITE under UVSW
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Pictures Galery:



 ...
...  Go to the galery (34 pictures)
Go to the galery (34 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Thermoluminescence: OUI
Comments:
A very thin coating of SCHEELITE on WOLFRAMITE makes it glow and give the impression that this mineral is luminescent. The tungstates of calcium, strontium, magnesium and zinc, and the molybdates of calcium are known to show luminescence upon excitation by cathode rays or short-wave ultra-violet radiation. It is commonly assumed that this luminescence is characteristic of the tungstate and molybdate groups. The reason why other tungstates and molybdates are found to be non-luminescent is probably the temperature-quenching (see Nature article by F. A. Kröger in 1947 in the bibliography).
Activator(s) and spectrum:
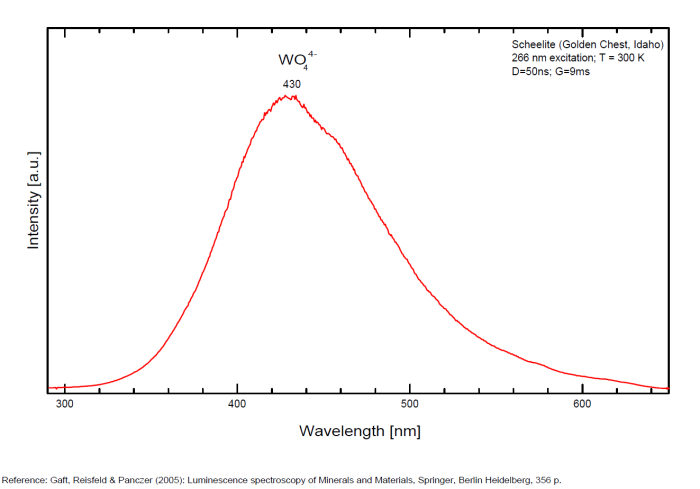
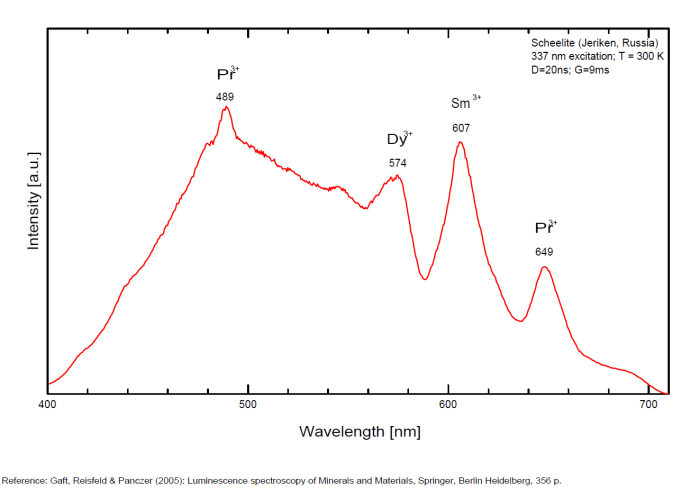
Activator(s): WO42-, Sm3+, Eu3+, Dy3+, Ho3+, Er3+, Tb3+, Pr3+, Nd3+, Yb3+, Tm3+,
Peaks in the spectrum (nm):
WO42-: Broad band centered at 425 - 435nm (Lifetime: 9μs @ 405nm)
Tb3+: 439nm
Dy3+: 488, 575nm
Sm3+: 609, 647nm
Pr3+: 607nm
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:
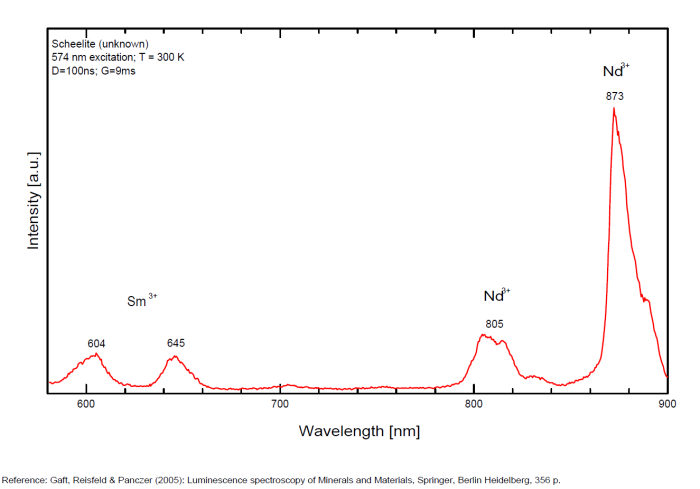 ...
...Comments on spectrum and activators:
Scheelite is characterized by broad luminescent bands centered at 425 - 435 nm (blue emission) of intrinsic activator (WO4)2- groups and impurity (MoO4)2- groups. Those broad bands are attributed to an intrinsic slight distortion of the [WO4]/ [MoO4] tetrahedral group. [WO4] lifetime: 9μs (@ 405nm). Such strong bands prevent in many cases the detection of lines of rare-earth elements, especially Tm, Er and Ho, which have weak luminescence in the corresponding spectral range. Yet, scheelite incorporates often tens to thousands of ppm RRE in substitution for Ca giving sometimes typical peaks in the fluorescence spectrum. Visible peaks in relation with the presence of REE: 488 et 575 (Dy3+), 609 et 647 (Sm3+), 439 (Tb3+) et 607 (Pr3+). But the lines of certain REE may be hidden by stronger luminescence of others REE. For example, the luminescence of Pr3+ is difficult to detect because its radiative transitions are hidden by the lines of Sm3+, Dy3+ and Nd3+, the luminescence of Tm3+ is concealed by Tb3+ and so on. The pegmatitic and hydrothermel scheelite shows the lines of Erbium and Terbium, while scheelite occurrences related to eruptive complex and sulfide ore shows dominantly the lines of the REE of the Cerium group. Under cw laser excitation at 532 several narrow lines have been found in red and IR part of the spectrum connected with Nd3+ and possibly Sm3+ centers. Green emission due to (MoO4)2-(Tarashchan) or possibly Pb (Blasse). The color of the fluorescence of scheelite gives an idea of the unwilled presence of molybdenium in the ore. Concentrate of scheelite not penalized for molybdenium have a distinct blue fluorescence color. Those that fluoresce white are borderline and contain roughly 0,35% to 1% of Mo. And scheelite that fluoresces distinctly yellow contains more than 1% corresponding to a transition to powellite. Higher than 4,8%, the yellow fluorescence color stays unchanged and cannot anymore be used as an indication of the percentage of Mo. Using this property, a method was developped by R.S. Canon jr. (1942) while studying tungsten deposits in the seven Devils mining district of Idaho. A serie of finely powdered synthetic preparation or natural ore of known composition are permanently mounted in circular areas on a black card, being placed in order of increasing molybdenium content. There are twelve standard values on the card: 0,05, 0,19, 0,33, 0,48, 0,72, 0,96, 1,4, 2,4, 3,4, and 4,8% plus a pure calcium molybdate (48% Mo). Alternating with the covered circle are circular holes of the same size. The card is used by placing a hole over a powdered sample of the scheelite ore to be tested and comparing the fluorescence color of the sample with those of the adjacent standards. The sample will be found to have a fluorescence color according or between two standards and hence the approximate composition could be defined.
Best localities for fluorescence (*):
- Pingwu beryl mine, Huya township, Mt Xuebaoding, Pingwu Co., Mianyang Prefecture, Sichuan Province, China;
- Yaogangxian Mine, Yaogangxian W-Sn ore field, Yizhang Co., Chenzhou Prefecture, Hunan Province, China;
- Huanggang Fe-Sn deposit, Hexigten Banner, Ulanhad League, Inner Mongolia Autonomous Region, China
- Mt. Xuebaoding, Sichuan Province, China (bright red MW);
- Xuebaoding Mountain, near Pingwu, Sichuan Province, China (bluish-white SW, orange LW);
- Gharmung area, Skardu District, Gilgit-Baltistan, Pakistan;
- Pari, Kharmang District, Gilgit-Baltistan, Pakistan;
- Tae Hwa Mine, Neungam-ri, Angseong-myeon, Chungju, Chungcheongbukdo, South Korea;
- Mundo Nuevo, Huamachuco, Sanchez Carrion Province, La Libertad, Peru;
- Morro Velho mine, Nova Lima, Minas Gerais, Brazil;
- Guadalupana Mine, Sierra del Chivato, Santa Cruz, Santa Cruz Municipality, Sonora, Mexico (weak Brownish-greenish SW);
- Cínovec / Zinnwald, Erzgebirge; Krusné Hory Mts, Germany & Czech Republic;
- Wolfram Bergbau und Hütten AG Mine (7 km S of Mittersill) Western ore field, Scheelite deposit, Felben valley, Hohe Tauern, Salzburg, Austria;
- Långban, Filipstad, Värmland, Sweden;
- Camp Bird Mine, Camp Bird, Sneffels District, Ouray, Ouray District, San Juan Mts, Ouray Co., Colorado, USA (blue SW, white LW);
- Kernville, Cove Mining District, Kern Co., California, USA;
- Route 20 construction site, Northborough, Worcester Co., Massachusetts, USA;
- Trumbull, Fairfield County, Connecticut, USA;
- Franklin Mining District, Sussex Co., New Jersey, USA (MW+SW white to cream);
- Carrock Mine, Mungrisdale, Eden, Cumbria, England, UK;
- Mont Chemin mining district, Martigny, Valais, Switzerland;
- Virgen de la Encina Mine, Salas de los Barrios, Ponferrada, León, Castile and Leon, Spain;
- Conchita Mine, Estepona, Málaga, Andalusia, Spain;
- Traversella Mine, Traversella, Metropolitan City of Turin, Piedmont, Italy;
- Kimmeria, Xanthi, East Macedonia and Thrace, Greece;
- Western ore field, Mittersill Scheelite deposit, Mittersill, Zell am See District, Salzburg, Austria;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Collecting Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2004, ISBN 0-7643-2091-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Ultraviolet Light and Fluorescent Minerals, Th. Warren, S. Gleason, R. Bostwick, et E. Verbeek, 1995, ISBN 0-9635098-0-2 ,
- Mt St Hilaire Website: http://www.saint-hilaire.ca ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- Luminescence properties of rare earth ions in fluorite, apatite and scheelite minerals, M. Czaja, S. Bodył, P. Głuchowski, Z. Mazurak and W. Strek, Journal of Alloys and Compounds Volume 451, Issues 1-2, 28 February 2008, Pages 290-292
- Der Aufschluss, Vol.48, n°2 March/April 1997 Langban minerals by Fritz Blatter;
- The Picking Table (Journal of the Franklin-Ogdensburg Mineralogical Society), vol. 55, no. 2, p. 13-25
- http://www.minerant.org/archief/fluo-Belgie.pdf
- Marvelous Midwave, Jeffrey Shallit, UV-Waves, vol.39, November-December 2009
- Electronic band structures of the scheelite materials CaMoO4, CaWO4, PbMoO4, and PbWO4, Y. Zhang, N. A. W. Holzwarth, and R. T. Williams, PHYSICAL REVIEW B VOLUME 57, NUMBER 20 15 MAY 1998
- Effect of the W:Mo ratio on the shift of excitation and emission spectra in the scheelite-powellites series, R. Michael Tyson, William R. Hemphill, Arnold F. Theisen, American Mineralogist, Volume 73, pages 1145-1154, 1988
- Luminescence properties of rare earth ions in fluorite, apatite and scheelite minerals, M. Czaja, S. Bodył, P. Głuchowski, Z. Mazurak and W. Strek, Journal of Alloys and Compounds Volume 451, Issues 1-2, 28 February 2008, Pages 290-292
- F. A. KRÖGER , Fluorescence of Tungstates and Molybdates,Nature,159, pages674–675(1947)
Images:
- Pingwu Beryl Mine, China: https://www.mindat.org/photo-905170.html
- Pingwu, China: https://www.mindat.org/photo-847491.html
- Cumbria, England, UK: https://www.mindat.org/photo-921211.html
- Zinnwald: https://www.mindat.org/photo-236693.html
- Massachusetts, USA: https://www.mindat.org/photo-52391.html
- Trumbull, Connecticut, USA: https://www.mindat.org/photo-159741.html
- Franklin, NJ, USA: https://www.mindat.org/photo-657482.html
- Camp Bird Mine, Colorado, USA: SW: https://www.mindat.org/photo-898441.html , LW: https://www.mindat.org/photo-898440.html
- Castile & Leon, Spain: https://www.mindat.org/photo-636178.html
- Tae Hwa Mine, South Korea: https://www.mindat.org/photo-484610.html
- Peru: https://www.mindat.org/photo-564694.html
- Gharmung area, Pakistan: https://www.mindat.org/photo-670952.html
- Pari, Pakistan: https://www.mindat.org/photo-1010331.html
- Mexico: https://www.mindat.org/photo-723804.html
- Traversella, Piedmont, Italy: https://www.mindat.org/photo-174875.html , https://www.mindat.org/photo-602595.html
- Brazil: https://www.mindat.org/photo-462264.html
- Mittersill, Austria: https://www.mindat.org/photo-734537.html
- China (red MW): https://www.mindat.org/photo-154258.html
Videos:
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Scheelite
http://www.mindat.org/show.php?name=Scheelite
 http://webmineral.com/data/Scheelite.shtml
http://webmineral.com/data/Scheelite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.