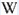Database of luminescent minerals
ANTHOPHYLLITE
Chemical formula: XMg7Si8O22(OH)2
Family: Silicates
Status: IMA-A (GP)
Crystal system : Orthorhombic
Display mineral: NON
Luminescence:
Longwave UV (365nm) colors: |
Violet Pink , | ||
Intensity LW:Strong | |||
Midwave UV (320nm) colors: |
Violet Pink , | ||
Shortwave UV (254nm) colors: |
Violet Pink , | ||
Intensity SW:Strong | |||
Shortwave (254nm) picture

Anthophyllite SW
Balmat Mine, Sylvia Lake, Fowler, St. Lawrence Co., New York, USA;
Photo & Col. © G. Barmarin
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Comments:
Material labelled tirodite from the International Talc Company mine, Talcville, New York, USA (Segeler, 1961), has been shown to be manganocummingtonite by electron microprobe (by Mike Hawkins, curator at the NY State Museum mineral collection). See further discussions at: http://www.mindat.org/mesg-7-206424.html Additional material labeled tirodite from the International Talc Company mine, Talcville, New York, USA, was confirmed to be anthophyllite by XRD performed by John Attard on a sample submitted by John Duck in April, 2012. Accordingly, without confirmation, the identity of similar material may be either manganocummingtonite or anthophyllite. Visual identification or identification by fluorescent response is not possible.
Activator(s) and spectrum:
Activator(s): Mn2+ ,
Peaks in the spectrum (nm):
band with a peak at 398nm, 513, 585nm Mn2+ repl. Mg2+ : band peaking at 651-655 nm

Anthophyllite, Balmat, NY, USA.
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
Comments on spectrum and activators:
The photoluminescence spectra, optical excitation spectra and PL decay curves of anthophyllite from Canada were obtained at 300 and 10 K by Aierken Sidike and Al.. The MnO content in the sample, determined using an electron probe microanalyzer, was high at 5.77 wt%. In the photoluminescence spectra obtained under 410nm excitation, bright red bands with peaks at 651 and 659nm were observed at 300 and 10 K, respectively. The origin of the red luminescence was ascribed to Mn2+ in anthophyllite from the analysis of the excitation spectra and photoluminescence decay times of 6,1–6,6 ms. In the PL spectra obtained under 240nm excitation at 300 K, a small violet band with a peak at 398 nm was observed. On the violet band at 10 K, a vibronic structure was observed. The origin of the violet luminescence was attributed to a minor impurity in anthophyllite.
(Source: Photoluminescence properties of anthophyllite, Aierken Sidike, Nuerrula Jilili, S. Kobayashi, K. Atobe and Nobuhiko Yamashita, Physics and Chemistry of Minerals, vol37 , 2009)
Best localities for fluorescence (*):
- International Talc Company Mine (Reynolds Mine), Talcville, St. Lawrence Co., New York, USA;
- Balmat Mine, Sylvia Lake, Fowler, St. Lawrence Co., New York, USA;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Reference for luminescence on the Internet:
Images:
- Talcville, New York, USA: http://www.mindat.org/photo-278698.html
- Talcville, New York, USA: http://www.mindat.org/photo-278693.html
- Nature s Rainbow
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Anthophyllite
http://www.mindat.org/show.php?name=Anthophyllite
 http://webmineral.com/data/Anthophyllite.shtml
http://webmineral.com/data/Anthophyllite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.