Database of luminescent minerals
LEUCOPHANITE
Chemical formula: (Ca,RRE)CaNa2Be2Si4O12(F,O)2
Family: Silicates
Status: IMA-GP
Crystal system : Orthorhombic
Display mineral: NON
Luminescence:
Longwave UV (365nm) colors: |
Violet Pink , Blue , Purple pink , | ||
Intensity LW:Strong | |||
Midwave UV (320nm) colors: |
Violet Pink , Purple pink , | ||
Intensity MW:Very Strong | |||
Shortwave UV (254nm) colors: |
Pink , Blue , Purple pink , | ||
Intensity SW:Strong | |||
Daylight picture

LEUCOPHANITE, Saga, Tvedalen, Norway;
Col. G.Barmarin; Photo: G. Barmarin
Longwave (365nm) picture

LEUCOPHANITE, Saga, Tvedalen, Norway;
UVLW
Col. G.Barmarin; Photo: G. Barmarin
Shortwave (254nm) picture

LEUCOPHANITE, Saga, Tvedalen, Norway;
UVSW
Col. G.Barmarin; Photo: G. Barmarin
Pictures Galery:



 ...
...Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Bluish | UV courts (254 nm): | Bluish |
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
To compare with meliphanite.
Activator(s) and spectrum:
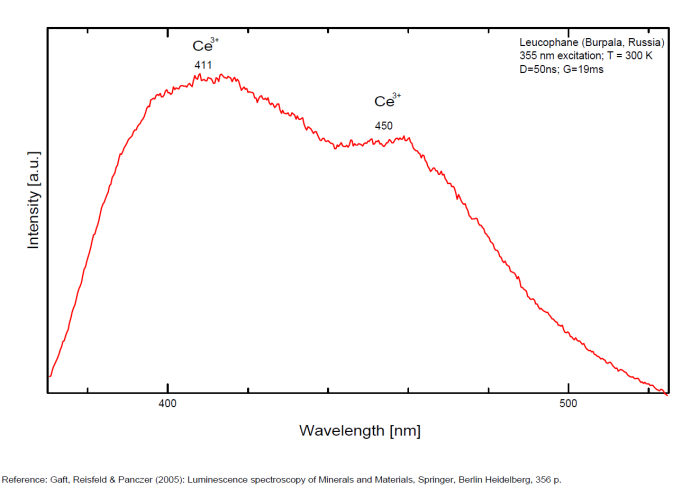
Activator(s): Ce3+, Eu2+, Sm3+, Dy3+, Tb3+, Mn2+ , Nd3+,
Peaks in the spectrum (nm):
Ce3+ repl. Ca2+ : 375, 411, 450nm
Eu2+ : 466-470nm
Tb3+ : 546nm
Sm3+: 607nm
Dy3+: 475, 488, 576nm
Nd3+: 885, 1060nm
Mn2+: 610nm
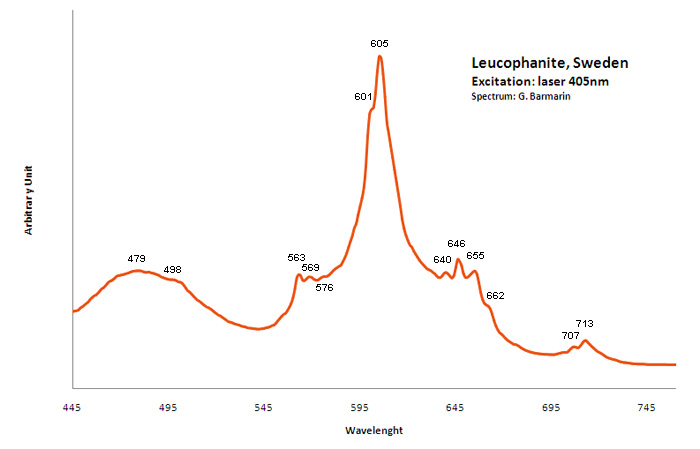
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:

 ...
...Comments on spectrum and activators:
Activators: a combinaison of RE elements - for exemple Ce3+ substituting to Ca (blue luminescence) and Mn (red luminescence) giving the magenta color (an orange flash typical of Mn(?) is seen on some samples). Cathodoluminescence: intense light-blue. The application of multiple forms of excitation (Friis et al. 2011) revealed that the UV-Blue emission in leucophanite and meliphanite consists of more than one emission center and is therefore more complex than previously thought. The most likely centers are defects related to the structure, e.g. in connection with the tetrahedral sites, and a Ce3+ centre. The difference in Na/Ca ratio between the two minerals make it possible for REE to substitute into two sites in meliphanite contrary, to just one in leucophanite. (Gaft)
Activators: Ce 3+, Eu 2+, Sm 3+, Dy 3+, Tb 3+, Nd 3+, Mn2+ substituting to Ca2+ (Gorobets in Gaft);
Best localities for fluorescence (*):
- Poudrette quarry, Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada (strong fluo SW);
- Bjorndalen Quarry, Sagasen Quarry and Saga I Quarry, Morge, Strandasen, Porsgrunn, east side of Langesundfjord, Larvik Plutonic Complex, Norway (strong fluo MW);
- Saga 1 Quarry, Sagåsen, Mørje, Porsgrunn, Telemark, Norway (strong fluo MW);
- Ilimaussaq complex, Nakalaq, Narsaq, Kitaa (West Greenland) Province, Greenland;
- Leucophanite occurrence, Kangerluarsuk Fjord, Ilímaussaq complex, Narsaq, Kujalleq, Greenland;
- Rasvumchorr Mt, Khibiny Massif, Kola Peninsula, Murmanskaja Oblast, Northern Region, Russia
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Mt St Hilaire Website: http://www.saint-hilaire.ca ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- Langesundsfjord - Wilfred Steffens
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2
- Ionoluminescence of Leucophanite, Henrik Friis, Adrian A. Finch, Peter D. Townsend, David E. Hole and Hassane El Mkami, American Mineralogist, February-March 2007 v. 92 no. 2-3 p. 254-260
Images:
- Langesundsfjorden, Norway (MW): http://www.mindat.org/photo-100780.html
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Leucophanite
http://www.mindat.org/show.php?name=Leucophanite
 http://webmineral.com/data/Leucophanite.shtml
http://webmineral.com/data/Leucophanite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.