Database of luminescent minerals
WILLEMITE
Chemical formula: Zn2SiO4
Family: Silicates
Status: IMA-GP
Crystal system : Hexagonal
Display mineral: OUI
Associated names (luminescent varieties, discredited names, synonyms, etc.): troostite, beta-willemite,
Luminescence:
Longwave UV (365nm) colors: |
Green , | ||
Intensity LW:Medium | Frequency LW:Rarely | ||
Midwave UV (320nm) colors: |
Green , | ||
Intensity MW:Strong | |||
Shortwave UV (254nm) colors: |
Green , | ||
Intensity SW:Very Strong | Frequency SW:Often | ||
Daylight picture

WILLEMITE, Franklin, New Jersey, USA;
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
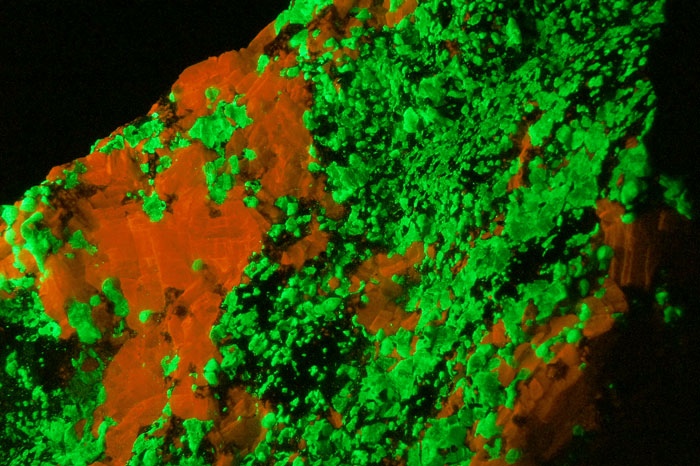
Shortwave (254nm) picture

WILLEMITE under UVSW, Franklin, New Jersey, USA;
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Pictures Galery:


 ...
...  Go to the galery (29 pictures)
Go to the galery (29 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Green | Strong | Often | UV courts (254 nm): | Green | Very Strong | Often |
Thermoluminescence: OUI
Comments:
Willemite was first recognized in New Jersey in 1822, although it had evidently been mined there for many years before. It was then known as silicious oxide of zinc. The name willemite was applied by A. Lévy in 1880 to what afterwards proved to be the same mineral. His material was found in the Netherlands, and was named after Willem I (William I )(1772-1844), King of the Netherlands. It came from the small (less than 1,400 acres) neutral state of Moresnet situated between Prussia and Belgium (though the present kingdom of Belgium was not founded until that year--1830). Under the Treaty of Versailles (1919) it is now in Belgium. In this connexion it is interesting to recall that the name belgite has been suggested for this mineral. R. Panebianco, writing in esperanto in 1916, objected to naming minerals after kings, preferring a name derived from the locality. He, however, overlooked the fact that the locality was not, at that time, in Belgium ! From: South African occurrences of willemite. Fluorescence of willemite and some other zinc minerals in ultra-violet rays. By L. J. Spencer, Keeper of Minerals in the British Museum (Natural History). 1927 The occurrenee of willemite at Broken Hill, Northern Rhodesia was first recorded by Prof. H. Buttgenbach in 1919 (H. Buttgenbach. La calamine des ossements fossiles de Broken-Hill, (Rhodésie). Ann. Soc. Géol. Relgique, 1919 vol. 42) Troostite : willémite containing manganese ; Prior to the development of halophosphor in 1942, the first generation of phosphor used in fluorescent tube was synthetic willemite activated with manganese-II.
Beta-willemite nom erroné appliqué a une variété trouvée à Franklin-Sterling Hill et fluorescente en jaune ;
Certains échantillons de Brandtite fluorescents en vert OC devraient leur fluorescence à la willemite associée ;
Activator(s) and spectrum:
Activator(s): Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ replacing Zn2+ : broad band peaking at +/- 525nm

Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Comments on spectrum and activators:
Lifetime: 400μs @520nm The action of ultra-violet rays on a large series of minerals of all kinds was tested by G. F. Kunz and C. Baskerville in 1903, and by E. Engelhardt in 1912. T. Liebisch in 1912 determined spectroscopically the nature of the green fluorescence from willemite. W.S. Andrews has pointed out in 1922 that artificially prepared willemite is only active when it contains some manganese, the shade of the green fluorescence depending on the amount of manganese present. Palache (1928) confirmed that the willemite containing manganese fluoresces the strongest. Dake (1941) stated that most connoisseurs of fluorescent minerals agree that willemite from Franklin an Ogdensburg, N.J. is the most spectacular of all luminescent minerals.
Best localities for fluorescence (*):
- Franklin Mine, Franklin, Sussex County, New Jersey, USA;
- Sterling Hill Mine, Ogdensburg, Sussex County, New Jersey, USA;
- Hogan Claim, near Wickenburg, Yavapai County, Arizona, USA (Green SW associated with calcite and fluorite);
- Desert View Mine, Holcomb Valley, San Bernardino County, California, USA (green SW associated with calcite red SW and sometimes hardystonite blue SW);
- Desert View Mine, Fawnskin (Grout), Bear Valley District, San Bernardino Mts, San Bernardino Co., California, USA;
- Purple Passion Mine, Red Picacho District, Wickenburg area, Yavapai Co., Arizona, USA;
- El Refugio Mine, Sinaloa, Mexico (green SW associated with hydrozincite blue-white SW)(+/-2007)
- Star Zinc Mine, Broken Hill, North Western province, Zambia;
- Excelsior prospect, Lusaka, Zambia;
- Hasselhojden, Grythyttan, Hällefors, Västmanland, Sweden;
- Garpenberg Norra Mine, Garpenberg, Hedemora, Dalarna, Sweden (green SW associated with calcite SW red pretty like Franklin specimens, with sometimes sphalerite SW yellow and hydrozincite SW blue);
- Berg Aukas, Grootfontein District, Otjozondjupa Region Namibia;
- Poudrette quarry, Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada (strongly phosphorescent, associated with serandite, calcite & rhodochrosite)
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Collecting Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2004, ISBN 0-7643-2091-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Ultraviolet Light and Fluorescent Minerals, Th. Warren, S. Gleason, R. Bostwick, et E. Verbeek, 1995, ISBN 0-9635098-0-2 ,
- Franklin Website: http://franklin-sterlinghill.com ,
- Mt St Hilaire Website: http://www.saint-hilaire.ca ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- THE MINERALS OF FRANKLIN AND STERLING HILL, NEW JERSEY, ALBERT S. WILKERSON, BULLETIN 65 NEW JERSEY GEOLOGICAL SURVEY, 1962
- Franklin Mining District (Mindat.org)
- Franklin Mine, Franklin, Sussex County, New Jersey, USA;
- Sterling Hill Mine, Ogdensburg, Sussex County, New Jersey, USA;
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2
- Der Aufschluss, Vol.48, n°2 March/April 1997 Langban minerals by Fritz Blatter;
- http://www.minersoc.org/pages/Archive-MM/Volume_21/21-119-388.pdf
- http://www.minerant.org/archief/fluo-Belgie.pdf
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
Images:
- Hasselhojden, Sweden: http://www.mindat.org/photo-174164.html
- Garpenberg Norra Mine, Sweden : https://www.mindat.org/photo-615793.html
- Desert View Mine, Ca, USA
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Willemite
http://www.mindat.org/show.php?name=Willemite
 http://webmineral.com/data/Willemite.shtml
http://webmineral.com/data/Willemite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.