Database of luminescent minerals
CALCITE
Chemical formula: CaCO3
Family: Carbonates
Status: IMA-GP
Crystal system : Rhomboedric
Display mineral: OUI
Associated names (luminescent varieties, discredited names, synonyms, etc.): manganocalcite, marbre, plumbocalcite, thinolite, glendonite, calcaire, strontiocalcite, cobaltocalcite, Petoskey Stone ,
Luminescence:
Longwave UV (365nm) colors: |
Pink , White , Bluish White , Yellowish White , Pinkish White , Yellow , Orangy yellow , Orange , Orange Red , Red , Violet red , Violet Pink , Salmon pink , Greenish Yellow , Blue , Violet , Greenish white , Violet blue , Purple pink , sky-blue , | ||
Intensity LW:Strong | Frequency LW:Often | ||
Midwave UV (320nm) colors: |
Red , White , Bluish White , Yellowish White , Pinkish White , Pale Yellow , Orange Red , Red , Violet red , Violet Pink , Pink , Salmon pink , Yellowish Green , Greenish Blue , Greenish white , Yellowish , sky-blue , | ||
Intensity MW:Strong | Frequency MW:Often | ||
Shortwave UV (254nm) colors: |
Blue , White , Bluish White , Yellowish White , Pinkish White , Pale Yellow , Orangy yellow , Orange , Orange Red , Red , Violet red , Violet Pink , Pink , Salmon pink , Green , Greenish , Bluish , Greenish white , Violet blue , Yellowish , Purple pink , sky-blue , | ||
Intensity SW:Strong | Frequency SW:Often | ||
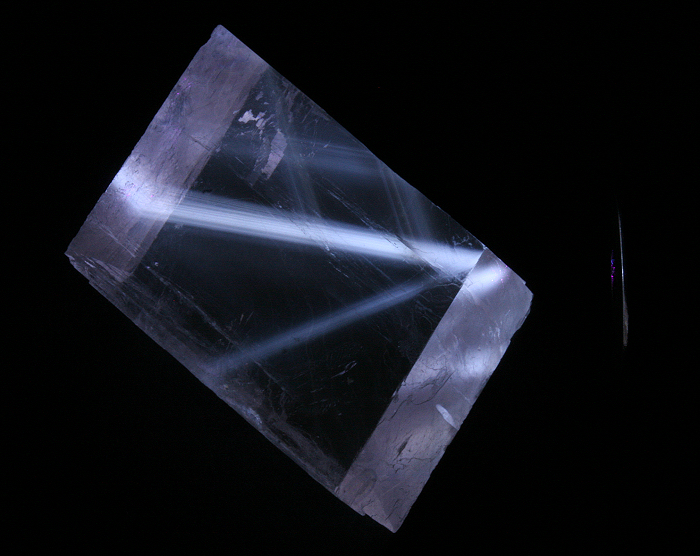
Daylight picture

Calcite, Nuova Leone.
Col. G. Barmarin; Photo: G. Barmarin
Longwave (365nm) picture

Calcite, Nuova Leone, OL (365 nm). Col. G. Barmarin; Photo: G. Barmarin
Midwave (330nm) picture

CALCITE under UVMW;
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Shortwave (254nm) picture

CALCITE,
Photo & Col. © G. Barmarin
Pictures Galery:


 ...
...  Go to the galery (68 pictures)
Go to the galery (68 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Greenish white | Strong | Often | UV courts (254 nm): | Bluish White | Strong | Often |
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
manganocalcite OC rose, rouge; OL : orange, rose, rouge, rouge orangé;
plumbocalcite : OC et OL: rouge sombre ;
strontiocalcite OL et OC: crème, blanc-jaunâtre, rose;
thinolite ( = pseudomorphose après gaylussite): OL et OC: orange, blanc bleuté;
Activator(s) and spectrum:
Activator(s): Mn2+ , Pb2+, ST (Singlet-triplet)-Matière organique en impureté, Ce3+, Sm3+, Eu3+, Dy3+, Tb3+, Nd3+, Centres dûs aux effets des radiations, Tm3+, PO2* , CO3-* ,
Peaks in the spectrum (nm):
Pb2+ repl. Ca2+ : band at 320-325nm (60nm half-width) Ce3+I : 350-355, 380nm (60nm half-width) Ce3+II : 400nm Mn2+II : 580 - 620nm ( band with 100nm half-width) Mn2+I repl. Ca2+ : 610 - 630nm (band) ST : 400-500nm and 450-550nm [CO3-]* intr. : 420nm (radiation induced CO33- ) [PO2]*? repl. [CO3] : 570nm Nd3+ : 817, 889nm Sm3+ : 614, 615, 626nm Dy3+ : 483, 577nm Tb3+ : 544, 545nm Eu3+I : 591, 618, 696, 709nm Eu3+II : 575nm Eu3+ : 592, 619nm Radiation induced centers : 415-420, 550nm (in pure Iceland spar)

Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum of CALCITE in CSV
You can download the CSV file by clicking here: fichier csv
Spectrum Galery:



 ...
...Comments on spectrum and activators:
In nature, RE and Mn2+ can substitute for Ca, becoming luminescence centers. Most calcite luminescence is attributed to Mn2+, while Mn2+ hides rare earth emission, and so far their luminescence in calcite is rather poorly characterized. Activator: Mn2+ substituting to Ca2+ (large peak around 610 - 630 nm); also Pb2+ replacing Ca2+ with a coordination number of 6 producing 312-325nm (Tarashchan) and radiation-induced (CO3)3- 403 nm (Kasyanenko); also RRE: Ce3+, Sm3+, Dy3+, Eu3+, Tb3+, Tm3+, Nd3+ (Gaft) Both luminescence centers are thermally unstable with the blue emission disappearing after heating at 500 °C, and the orange emission disappearing after heating at different temperatures starting from 230 °C, although sometimes it is stable up to 500 °C in different samples. Stokes (1852) was unable to observe the fluorescence of calcite. In 1859, Becquerel found that fluorescent calcite contained up to 2,7% Mn. In 1867 he studied calcite with his new phosphoroscope measuring a 0,5s for phosphorescence. Lommel (1884) studied calcite using sun as UV source and in 1909, Pochettino employed cathode ray to study the fluorescence of calcite. Sohncke (1896) while investigating polarized fluorescence found calcite from Iceland showing a marked fluorescence. Nichols, Howes and Wilbur were of opinion that Mn traces cause the luminescence of calcite and demonstrated this with certainty in later investigations. Tanaka working in the Cornell Laboratory (1924) also concluded that the chief activator in calcite, crystalline limestone and dolomite was manganese. Pringsheim (1928) found also Mn as the most important activator in calcite. In 1939, G. Fonda made the synthesis of fluorescent calcite by precipitation of calcium carbonate in presence of a manganese salt. But the product is not exactly as calcite in nature. Another technic used to obtain fluorescent calcite is to firing a small amount of manganese salt (about 0,005%) in calcium carbonate for about one hour at 1000° (in an atmosphere of carbon dioxide). Another successful synthesis of luminescent calcite, closely resembling the salmon red fluorescing calcite of Franklin is achieved by precipitating the carbonate from a mixture of calcium and manganese chlorides by ammonium carbonate under special conditions. The optimum concentration for the dilution of the chlorides is 1Mn:30Ca. Dilute solutions containing 1,8% CaCl2.6H2O and 1,5%(NH4)2CO3.H2O, with 50% excess ammonium carbonate have been found best. The precipitation is carried out at about 70°C, followed by holding at room temperature for several weeks before filtering, boiling for 10 minutes gives similar results. With this mode of preparation, synthetic calcite has 30% the intensity of Franklin calcite. (text from Fluorescent Gems and Minerals by Jack De Ment, 1947) In 1943, De Ment identified the uranium luminescence spectrum of calcite from Deming, NM, USA under SW and the Terlingua, Texas, USA calcite. For Terlingua calcite, he found a strong band at 410-450nm (purple-blue), a bright green band at 450-545nm and a weak reddish band at 545-620nm. The blue long-lived phosphorescence have the same spectral bands as fluorescence. De Ment mentioned in 1947 the role of RRE as activator in calcite (Dy, Sm, Y, Ce) and of Thallium (?). Cathodoluminescence: light-blue, orange;
Natural calcite from Kuerle, Xinjiang, China, shows orange-red fluorescence when exposed to short-wave ultraviolet (UV) light (Hg 253.7nm).
The PL emission spectrum under 208 nm excitation consists of three bands: two UV bands at 325 and 355 nm and an orange-red band at 620 nm.
The three bands are ascribed to Pb2+, Ce3+ and Mn2+, respectively, as activators.
The Pb2+ excitation band is observed at 243 nm, and the Ce3+ excitation band at 295 nm.
The Pb2+ excitation band is also observed by monitoring the Ce3+ fluorescence, and the Pb2+ and Ce3+ excitation bands, in addition to six Mn2+ excitation bands,
These indicate that four types of the energy transfer can occur in calcite through the following processes:
(1) Pb2+ → Ce3+,
(2) Pb2+ → Mn2+,
(3) Ce3+ → Mn2+ and
(4) Pb2+ → Ce3+ → Mn2+.
(Source:Energy transfer among Pb, Ce and Mn in fluorescent calcite from Kuerle, Xinjiang, China,by Aierken Sidike, X.-M. Wang, Alifu Sawuti, H.-J. Zhu , I. Kusachi and N. Yamashita in Revue Physics and Chemistry of Minerals,Vol.33, Nov.2006, see link below)
The unusual luminescence of particular varieties of natural pink calcite (CaCO3) samples (Terlingua type) was studied by laser-induced time-resolved luminescence spectroscopy at different temperatures.
The luminescence is characterized by intense blue emission under shortwave UV lamp excitation with an extremely long decay time, accompanied by pink-orange luminescence under longwave UV excitation. This calcite contains negligible quantities of impurities, which may be potentially connected to such luminescence.
Investigation including optical absorption, natural thermostimulated luminescence (NTL) and Laser-Induced Breakdown Spectroscopy (LIBS) studies conduct to 2 luminescence centers: a narrow violet band, with max = 412 nm, FWHM= 45 nm, two decay components of 1 = 5 ns and 2 = 7.2 ms, accompanied by very long afterglow, and an orange emission band with max = 595 nm, FWHM= 90 nm, and = 5 ns.
Both centers have spectral-kinetic properties very unusual for mineral luminescence, which in combination with extremely low impurity concentrations prevent their identification with specific impurity related emission.
The most likely explanation of these observations may be the presence of radiation-induced luminescence centers. The long violet afterglow is evidently connected with trapped charge carrier liberation, with their subsequent migration through the valence band and ultimate recombination with a radiation-induced center responsible for the unusual violet luminescence.
(Source: The nature of unusual luminescence in natural calcite CaCO3, Michael Gaft, Lev Nagli, Gerard Panczer, Glenn Waychunas and Naomi Porat, American Mineralogist; January; v. 93; no. 1; p. 158-167, 2008 see link below)
Most common orange-red fluorescence is due to divalent manganese, with a co-activator to absorb the UV and transfer the energy to the manganese. Most common co-activator is probably lead. Cerium can act as a co-activator, suspected when the fluorescence is stronger with long-wave UV. Magnesium in the calcite will shift the spectrum a bit to the red side. Some calcites fluoresce closer to orange. Both trivalent dysprosium and trivalent samarium are said to be capable of producing orange fluorescence. Pink or yellow fluorescence with a greenish phosphorescence is suspected to be caused by organic activators. Green may be due to interstitial inclusion of mineral activated by uranium (often hyalite).
Best localities for fluorescence (*):
- Baelo-Claudia San Bartolome quarry, Tarifa, Cádiz, Andalusia, Spain (unusual green LW);
- Calcite pseudomorph after glauberite, Camp verde, Yavapai County, Arizona, USA (fluo SW bluish white);
- Terlingua-type: San Vicente Mine, Boquillas Del Carmen, Ocampo Municipality, Coahuila, Mexico;
- Little 38 Mine, Terlingua Mercury Mines Zone, Terlingua District, Brewster Co., Texas, USA;
- Buckwheat dump mineral collecting site, Franklin, Franklin Mining District, Sussex Co., New Jersey, USA (fluo red SW a classical from Franklin);
- Iraí, Alto Uruguai region, Rio Grande do Sul, Brazil ;
- Calcite in fossilized clamshell (Mercenaria Permanga), Ruck s Pit, Fort Drum, Okeechobee County, Florida, USA;
- Challenger Cave, Monterrey, Nuevo Leon, Mexico (Double refraction clivage rhomboedra);
- Ajax Mine, Near Superior, Arizona, USA (Calcite + hydrozincite);
- Near Silver Bell Mine, Pima County, Arizona, USA (calcite + fluorite);
- locality near Lovington, New Mexico, USA (green SW);
- Idarado Mine, Telluride, Ouray District, San Miguel Co., Colorado, USA (bright orange);
- Woodward Ranch, Alpine, Brewster Co., Texas, USA (red SW);
- Annabel Lee Mine, Harris Creek Mining Sub-District, Hardin Co., Illinois, USA (LW red but not spectacular)
- Naica, Mun. de Saucillo, Chihuahua, Mexico (red SW);
- Guanajuato, Mun. de Guanajuato, Guanajuato, Mexico (red SW);
- Melchor Múzquiz, Mun. de Melchor Múzquiz, Coahuila, Mexico (pink LW, blue SW);
- Aghbar Mine, Aghbar, Bou Azer District, Tazenakht, Ouarzazate Province, Souss-Massa-Draâ Region, Morocco (pink-violet calcite with Intense fluorescence red-orange SW & LW);
- Wessels Mine, Hotazel, Kalahari manganese field, Northern Cape Province, South Africa (red-orange);
- N Chwaning Mines, Kuruman, Kalahari manganese field, Northern Cape Province, South Africa (red, red-orange);
- Tsumeb Mine, Tsumeb, Otjikoto Region, Namibia
- Dal negorsk, Kavalerovo Mining District, Primorskiy Kray, Far-Eastern Region, Russia (pink, orange, red-orange);
- Pachapaqui District, Bolognesi Province, Ancash Department, Peru (red);
- Boldut Mine, Cavnic, MaramureÈ™ Co., Romania (SW+LW);
- Droujba Mine, Djurkovo Complex, Laki, Laki Obshtina, Rhodope Mts, Plovdiv Oblast, Bulgaria (red);
- Mogilata deposit, Septemvri mine, Madan ore field, Rhodope Mts, Smolyan Oblast, Bulgaria (red);
- Knolle mine, Åmål, Dalsland, Sweden (deep red SW);
- Fuzichong Pb-Zn-Ag ore field, Cenxi Co., Wuzhou Prefecture, Guangxi Zhuang Autonomous Region, China (pink-red);
- Fengjiashan Mine, Daye Co., Huangshi Prefecture, Hubei Province, China (red);
- Chenzhou Prefecture, Hunan Province, China (pink);
- Huanggang Fe-Sn deposit, Hexigten Banner, Ulanhad League, Inner Mongolia Autonomous Region, China (red-orange);
- La Serrata (Maria de Cerrate), Lorca, Murcia, Spain (pink LW, white SW+phospho);
- Langban, Filipstads Kommun, Värmlands län, Sweden (Mn bearing calcite);
- Äußerer Knorrkogel, Gschlöss valley, Tauern valley, East Tyrol, Tyrol, Austria (calcite crystals showing a red fluorescence due to a phorphyrine complex content);
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Collecting Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2004, ISBN 0-7643-2091-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Ultraviolet Light and Fluorescent Minerals, Th. Warren, S. Gleason, R. Bostwick, et E. Verbeek, 1995, ISBN 0-9635098-0-2 ,
- Franklin Website: http://franklin-sterlinghill.com ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2.
- Terlingua Calcite website by FMS member Fred Gossien
- Terlingua calciet, Geonieuws, November 2010
- Energy transfer among Pb, Ce and Mn in fluorescent calcite from Kuerle, Xinjiang, China,by Aierken Sidike, X.-M. Wang, Alifu Sawuti, H.-J. Zhu , I. Kusachi and N. Yamashita in Revue Physics and Chemistry of Minerals,Vol.33, Nov.2006
- ACTIVATORS OF LUMINESCENCE IN SPELEOTHEMS AS SOURCE OF MAJOR MISTAKES IN INTERPRETATION OF LUMINESCENT PALEOCLIMATIC RECORDS, Y. Y. Shopov, Int. J. Speleol., 33 (1/4), 2004: 25-33
- The nature of unusual luminescence in natural calcite CaCO3, Michael Gaft, Lev Nagli, Gerard Panczer, Glenn Waychunas an Naomi Porat, American Mineralogist; January; v. 93; no. 1; p. 158-167, 2008
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
Images:
- Baelo-Claudia San Bartolome quarry, Andalusia, Spain (unusual green LW): http://www.mindat.org/photo-469451.html
- La Serrata (Maria de Cerrate), Lorca, Murcia, Spain: https://www.mindat.org/photo-441591.html
- Baelo-Claudia San Bartolome quarry, Andalusia, Spain (various wavelenght): https://www.mindat.org/photo-789474.html, phospho: https://www.mindat.org/photo-789480.html
- Bou Azer, Ouarzazate Province, Morocco: http://www.mindat.org/photo-245560.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-240243.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-229819.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-345156.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-173733.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-120787.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-167990.html
- Wessels Mine, Hotazel, South Africa: http://www.mindat.org/photo-251458.html
- Wessels Mine, Hotazel, South Africa (blue-white LW): http://www.mindat.org/photo-251500.html
- idem SW: http://www.mindat.org/photo-251501.html
- N Chwaning Mines, South Africa: http://www.mindat.org/photo-201321.html
- N Chwaning Mines, South Africa: http://www.mindat.org/photo-417566.html
- Tsumeb Mine, Namibia: http://www.mindat.org/photo-282345.html
- Dalnegorsk, Russia: http://www.mindat.org/photo-530313.html
- Peru: http://www.mindat.org/photo-552007.html
- Buckwheat dump mineral collecting site, Franklin, USA: http://www.mindat.org/photo-50789.html
- Woodward Ranch, Alpine, Texas, USA (showing double image of text): http://www.mindat.org/photo-161282.html
- Woodward Ranch, Alpine, Texas, USA: http://www.mindat.org/photo-359101.html
- Terlingua District, Brewster Co., Texas, USA (SW): http://www.mindat.org/photo-234087.html
- Terlingua District, Brewster Co., Texas, USA (LW): http://www.mindat.org/photo-234088.html
- Terlingua District, Brewster Co., Texas, USA (SW): http://www.mindat.org/photo-230289.html
- Terlingua District, Brewster Co., Texas, USA (LW): http://www.mindat.org/photo-230290.html
- Terlingua District, Brewster Co., Texas, USA (LW): http://www.mindat.org/photo-357255.html
- Terlingua District, Brewster Co., Texas, USA (SW): http://www.mindat.org/photo-357260.html
- Rucks pit, Florida, USA (clam fossil SW): http://www.mindat.org/photo-271150.html
- Naica, Mexico: http://www.mindat.org/photo-307066.html
- Guanajuato, Mexico: http://www.mindat.org/photo-376909.html
- Melchor Múzquiz, Coahuila, Mexico:
- Droujba Mine, Bulgaria: http://www.mindat.org/photo-237643.html
- Madan ore field, Bulgaria: http://www.mindat.org/photo-320925.html
- Madan ore field, Bulgaria: http://www.mindat.org/photo-320927.html
- Knolle mine, Sweden: http://www.mindat.org/photo-245378.html
- Fengjiashan Mine, Hubei Province, China: http://www.mindat.org/photo-407739.html
- Fuzichong Pb-Zn-Ag ore field, Guangxi Zhuang, China http://www.mindat.org/photo-407741.html
- Chenzhou Prefecture, Hunan Province, China: http://www.mindat.org/photo-429517.html
- Huanggang Fe-Sn deposit, Inner Mongolia, China: http://www.mindat.org/photo-447207.html
- Iraí, Rio Grande do Sul, Brazil: http://www.mindat.org/photo-407769.html
- Iraí, Rio Grande do Sul, Brazil: https://www.mindat.org/photo-632289.html
- Annabel Lee Mine, Illinois, USA (red LW): https://www.mindat.org/photo-435696.html
- Indiana, USA: https://www.mindat.org/photo-328713.html
Videos:
- calcite fluorescing pink/magenta: https://www.instagram.com/p/B5KxQT4n88N
- Calcite phosphorescence:
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Calcite
http://www.mindat.org/show.php?name=Calcite
 http://webmineral.com/data/Calcite.shtml
http://webmineral.com/data/Calcite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.